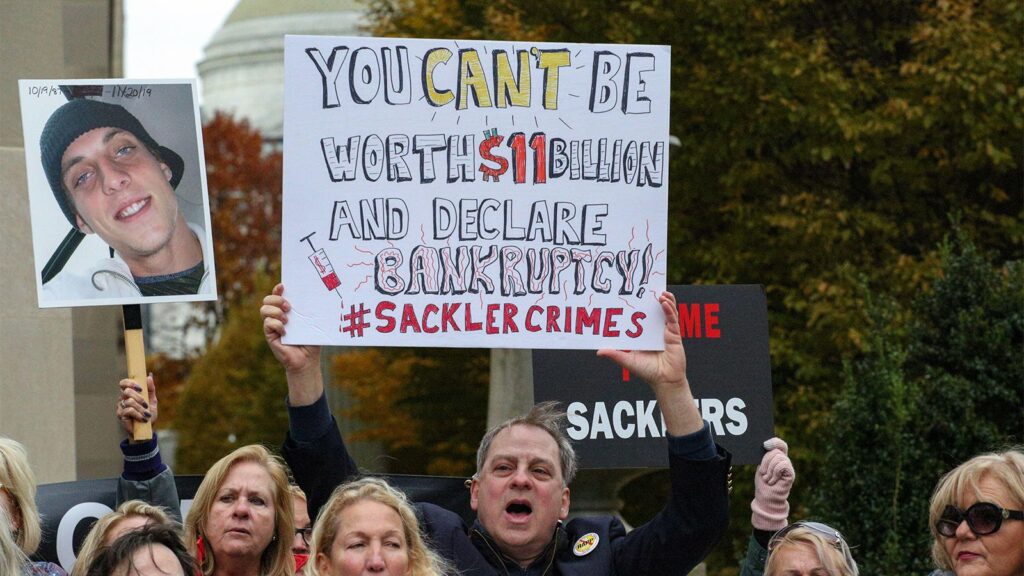LaBelle is an expert in addiction policy and drug control law. Fields is a legal fellow.
In March 2022, three members of the Sackler family listened as more than two dozen people on Zoom addressed them in a federal bankruptcy court in New York. For over 2 hours, the speakers talked about their own struggles with opioid use disorder and the loved ones lost to an overdose involving the opioid product OxyContin. They gave voice to their loss and their ongoing grief. Most of all, they expressed their anger at the Sacklers and Purdue Pharma (“Purdue”), the company that manufactured and marketed OxyContin, for the role they played in driving the opioid overdose epidemic, an epidemic that has taken over 500,000 lives in the U.S. since 2000.
While the hearing was intended to mark the end of a long process that began when Purdue filed bankruptcy in 2019, the legal wrangling over the Purdue bankruptcy continues today. In December 2023, the Supreme Court heard arguments in Purdue’s bankruptcy case, Harrington v. Purdue Pharma L.P.
The outcome of the Supreme Court review is pending. However, victims should not be made to wait even longer for relief. The Sackler family, which did not file for bankruptcy, can and should voluntarily establish a fund compensating those who have been injured due to Purdue’s marketing of OxyContin. Individuals with personal injury claims could then decide whether to release the Sacklers from future claims. Doing so will not undo the damage wrought by the opioid overdose epidemic, but it will help those who have been waiting years for closure.
The Current Case Against Purdue
Two branches of the Sackler family ran Purdue, and family members served on the company’s board until 2019. OxyContin, the long-acting opioid that entered the market in 1996, played a significant role in overdose deaths in the U.S. over the course of the opioid epidemic. The company is alleged to have used aggressive tactics to market the opioid despite knowledge of its addictive qualities. As a result, thousands of individuals, governments, and other entities sued Purdue. In the face of this blizzard of lawsuits, the company filed for bankruptcy in 2019, thereby halting lawsuits until a restructuring plan to repay creditors was approved.
The restructuring plan precludes anyone from suing a third party for actions resulting from the sale and distribution of OxyContin by Purdue, thereby releasing individual members of the Sackler family from future civil lawsuits. This bankruptcy provision is known as a “non-consensual third-party release.” The restructuring plan was eventually appealed to the U.S. Court of Appeals for the Second Circuit, which subsequently approved the restructuring plan. In 2023, the U.S. Department of Justice (DOJ) sought review to the Supreme Court on the issue of third-party immunity in bankruptcy.
Bankruptcy courts and federal circuit courts are split on the legal issue of non-consensual third-party releases, which often protect wealthy actors from liability. Given the lack of consistency amongst the circuits, bankruptcy experts have pleaded with Congress for bankruptcy reform and for the Supreme Court to establish uniformity on the issue of bankruptcy in cases like the one at hand. In the 117th Congress, the Nondebtor Release Prohibition Act of 2021 was introduced. The proposed legislation, prohibiting non-consensual third-party releases in bankruptcy court, failed to make it out of committee. The pending Supreme Court case may resolve this aspect of bankruptcy law.
Accountability, Purdue, and the Sackler Family
The family-owned company, Purdue, has made the Sacklers one of the wealthiest families in the U.S., with a collective worth estimated at $11 billion. It has also been reported that the Sacklers withdrew nearly $11 billion from the company beginning in 2008.
While no member of the Sackler family has been criminally charged in connection with the marketing of OxyContin, Purdue has been found criminally liable for its role, and civil allegations have been settled. On October 21, 2020, the DOJ announced that Purdue pled guilty in criminal court to multiple felonies for conspiracy to defraud the U.S. and violate the Food, Drug, and Cosmetic Act, and conspiracy to violate the Federal Anti-Kickback Statute. DOJ’s civil suits against Purdue and certain members of the Sackler family were also settled, with no determination of fault. Resolutions to these actions include the dissolution of the company and a total fine of $6 billion.
Prior to the DOJ’s actions, the number of people affected by Purdue’s marketing practices who brought individual claims against the company increased sharply after Kentucky filed a lawsuit against Purdue in 2007. Not all of these claimants agree with DOJ’s decision to seek Supreme Court review of the bankruptcy deal. In fact, over 60,000 of the individual claimants filed a brief to the Supreme Court supporting the bankruptcy agreement and opposing the DOJ petition for review.
Now, over 100,000 individual plaintiffs with personal injury claims must wait for the Supreme Court to decide this case before they receive recompense for harms associated with Purdue’s actions. The Sacklers have placed victims in an untenable position: to be certain they will receive any compensation, they must agree to shield the family from any future civil liability.
Aiding the Injured — Today
The Purdue bankruptcy plan includes a $700 million-to-$750 million fund to pay individuals who have personal injury claims against Purdue. If the Supreme Court rules that the plan can move forward, the amounts expected to be paid out to individuals range from $3,500 to $48,000.
Since Purdue has filed bankruptcy and individual members of the Sackler family have not, the family could voluntarily establish a fund to compensate people harmed by Purdue’s marketing of OxyContin, and allow individuals to choose to release future claims. However, it appears that the family’s insistence that they bear no responsibility for the overdose deaths involving OxyContin, and their desire to be shielded from future civil litigation, will prevent this from happening. Furthermore, while a $750 million fund is significant for the people who suffered due to Purdue’s marketing practices, it is a paltry amount in the grand scheme of the Sackler family fortune and the devastation wrought by the overdose epidemic.
The families and communities left behind deserve better. Nothing is stopping the Sacklers from doing the right thing and providing relief today. However, as Connecticut Attorney General William Tong said, “No settlement will ever come close to addressing the magnitude of suffering and harm caused by Purdue and the Sackler family…”
Regina LaBelle, JD, is the former acting director of the White House Office of National Drug Control Policy. She is currently a Distinguished Scholar at Georgetown University where she teaches in the MS in Addiction Policy and Practice program. Madison Fields, JD, is a fellow at The O’Neill Institute at Georgetown Law in Washington.
Please enable JavaScript to view the