
Monoclonal antibodies (or mAbs) are a class of therapeutic proteins designed to target specific molecules or cells in the body. They are used in treating cancer, autoimmune disorders, and infectious diseases, offering targeted therapies with fewer side effects compared to traditional treatments.
Monoclonal antibodies currently make up 14% of the overall active pharmaceutical pipeline, as tracked in GlobalData’s Drugs database. The majority of those candidates are in the discovery or preclinical stages of development, and 55% of them are for oncology.[2] The number of initiated clinical trials for mAbs has grown significantly over the past decade. In 2013, 1,232 trials were initiated. By 2018, this increased 86% to 2,286. In 2023, 3,270 new mAbs trials commenced.[3]
Many mAbs are filled into injectable presentations that require sterile filtration and aseptic filling. These drug substances also tend to have some product sensitivities that can complicate the process.
The typical fill finish process
A typical fill finish process will involve three major steps: formulation, sterile filtration, and aseptic filling.
Formulation refers to the process of combining different active pharmaceutical ingredients (APIs) and excipients to create a specific drug product. It involves determining the precise composition, concentrations, and ratios of the ingredients to achieve the desired therapeutic effect.
Sterile filtration is a critical step in pharmaceutical manufacturing that involves removing microorganisms from the drug product to ensure the final product is sterile. A 0.22-micron filter is used to capture bacteria, fungi, and other contaminants. The process involves passing the fluid through the filtration system, which typically consists of two sterile filters. Sterile filtration is commonly used for mAbs because this drug substance is heat-sensitive and cannot withstand terminal sterilization.
Aseptic filling is the process of transferring a sterile drug product into its final container, such as a vial, while maintaining aseptic conditions. It involves a series of controlled steps to prevent microbial contamination. The process typically includes sterilizing the containers, filling them with the sterile product using automated equipment inside an aseptic environment such as an isolator, sealing the containers, and performing appropriate inspections. Aseptic filling is crucial for preserving the sterility and integrity of the drug product and ensuring its safety and efficacy for patient use. It requires a specialized facility, equipment, and trained personnel to maintain strict aseptic techniques and minimize the risk of contamination throughout the process.
Challenges of processing mAbs
mAbs often have several product sensitivities that need to be managed during the lifecycle of the fill finish process – from storage to formulation to aseptic filling.
Here are some of the challenges this class of therapeutic brings:
- Temperature sensitivity – mAbs often require frozen storage conditions. This will need to be managed during formulation, filtration, and filling. These products cannot be exposed to room temperature for extended periods of time.
- pH sensitivity – it is critical to control the pH of the formulation to ensure it does not exceed or fall below the acceptable pH range. mAbs are quick to degrade in very high or low pH ranges.
- Prone to foaming – mAbs are likely to foam from physical agitation, like mixing. Reducing shear during mixing will be critical to prevent this from occurring.
- Low freeze-thaw stability – mAbs are prone to degradation from freeze-thaw cycles. This will be critical to manage for the drug substance solution that needs to be thawed prior to aseptic filling.
Formulation and filling solutions for mAbs
While mAbs do have certain product sensitivities and challenges, they are not a difficult therapeutic to process for a fill finish CDMO that is experienced in dealing with highly sensitive drug products. There are solutions to overcome each challenge that mAbs presents, and many of these solutions are standard applications to other highly sensitive APIs.
Overcoming temperature sensitivity
Many mAbs are prepared in a buffer solution at the API-manufacturing site and will arrive at the fill finish CDMO in solution. The solution arrives frozen and requires thawing prior to formulation. To limit exposure to room temperature, the drug substance is moved to a 2 – 8°C temperature-controlled unit (TCU) to thaw. Once thawed, the drug substance is moved to the cleanroom, and transferred into a jacketed, glass mixing vessel, connected to a water bath that circulates water through the jacket and controls the temperature to 2 – 8°C.
The drug product will be formulated in the jacketed mixing vessel (typically, this involves a simple dilution with buffer), and then sterile filtered into second sterile jacketed mixing vessel. This vessel will also be connected to a water bath to control the temperature of the vessel and keep drug product cool.
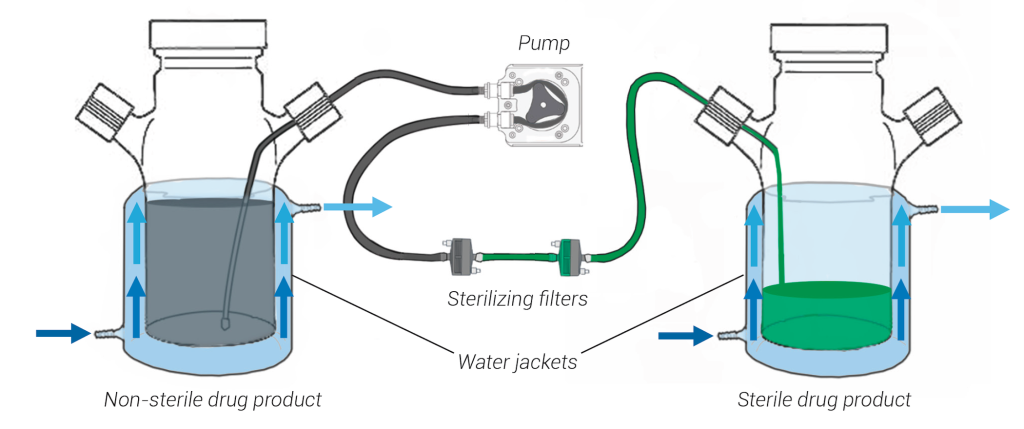
The vessel will be connected to the filling line, and units will be filled within an isolator, where the interior is kept at controlled room temperature. After the drug product is filled, it is moved to a 2 – 8°C work-in-progress TCU until it is pulled for visual inspection (VI).
For extremely temperature-sensitive drug product, filled product can be immediately transferred to the visual inspection suite for STAT VI. This expedites the process to ensure drug product is transferred to frozen storage conditions as soon as possible after filling.
Overcoming temperature sensitivity through lyophilization
For some mAbs projects, the stability of the drug product is greatly improved through lyophilization. This is the process of removing water and freeze-drying the drug substance to enhance shelf life. Lyophilization often eliminates the requirement for frozen storage conditions post-filling, thus simplifying the storage and distribution of the drug product and offering convenience and cost-savings to the sponsor.
Lyophilized mAbs will require a temperature-controlled thaw, filter, and fill, but once the product is filled into vials, it is moved into a lyophilizer for processing.
The lyophilization cycle should be optimized to reduce cycle time and enhance cake quality, and some CDMOs, such as Sharp Sterile Manufacturing (formerly Berkshire Sterile Manufacturing), may offer this service to best serve your project requirements.
Maintaining pH
Though the ideal pH range is specific to each unique mAb, most mAbs perform well in weakly acidic pHs. It will be critical to measure and maintain the acceptable pH range during formulating. The formulation team should monitor the pH at various stages of the process as they add buffer to dilute the drug product or make pH adjustments.
Reducing foaming
Foaming typically occurs when high mixing speeds with high shear are used in formulation. The best way to reduce foaming is to use a low-shear mixer and to mix slowly. At Sharp Sterile, we often employ an overhead, U-shaped mixing shaft for mAbs formulations.
Preventing degradation during a thaw
Several mAbs that we have worked with have demonstrated low freeze-thaw stability. If thawing is prolonged, then API may experience a concentration or pH gradients. For these projects, it is critical to ensure the thawing process occurs quickly without exposing the drug product to high temperature (e.g. room temperature) or variation in pH and concentrations. This is often achieved by periodically inverting the solution while it is thawing to mix the API to speed up thawing and to achieve better homogeneity. This also prevents cooler API from sitting next to the frozen core, where it can re-freeze, and it also moves warmer API solution from the outside of the bottle to the center where it can offer greater heat transfer to thaw the drug substance faster. mAbs can be shear-sensitive, however, so this inversion must be performed in a delicate manner.
Conclusion
While monoclonal antibody (mAbs) therapeutics pose unique challenges in the fill finish process, such as their sensitivity to pH fluctuations, temperature variations, freeze-thaw cycles, and their tendency to foam when mixed, these hurdles can be successfully overcome through meticulous attention and the implementation of suitable process modifications.
About Sharp’s services
Sharp is a leader in pharmaceutical packaging, clinical trial supply services and small-scale sterile manufacturing. For 70+ years, we’ve provided solutions to pharma and biotech clients from phase I trials through to commercial launch and lifecycle management. With facilities in the United States, United Kingdom, Belgium and the Netherlands and 30+ clinical depots globally, covering every region of the world, our experience is your strength.
[1] New Drug Approvals and Their Contract Manufacture: 2024 Edition
[2] GlobalData Pharmaceutical Intelligence Center. Drugs database.
[3] GlobalData Pharmaceutical Intelligence Center. Clinical Trials database.

Sign up for our daily news round-up!
Give your business an edge with our leading industry insights.
