Opioids are synthetic or natural agents that stimulate opioid receptors in the brain and nervous system and are often used to treat pain. However, the effects of the drugs can also lead to a high risk of substance abuse in some individuals. Opioid use disorder (OUD) is a chronic neurological disorder characterised by the compulsive, repeated use of opioid drugs, leading to prolonged self-administration. As the brain adapts to prolonged opioid use, the patient’s tolerance increases, so a higher dose of opioid is needed to stimulate the mesolimbic system in the same way as the original dose, causing patients to continue using higher doses of the drug. There is no single cause of OUD, but rather a combination of genetic and environmental factors, including neurological comorbidities such as depression. Opioid agonist maintenance therapy is the primary treatment approach for most OUD patients. It involves substituting opioids with agonists such as methadone, morphine, or medical-grade heroin, or partial agonists such as buprenorphine.
In August 2024, 110 high-prescribing psychiatrists, addiction specialists, neurologists, and primary care physicians from eight major markets (the US, France, Germany, Italy, Spain, the UK, Australia, and Canada) were surveyed by GlobalData on the key unmet needs in the treatment of OUD. Non-opioid medications that treat addiction and withdrawal, and drugs with improved safety and tolerability profiles, were consistently ranked by respondents as the most important unmet needs in the OUD market (ranked from 1 to 9, where 1 indicates the most significant need and 9 the least important). This trend was seen throughout eight major markets covered in the survey. Figure 1 shows the average ranking from the 110 respondents.
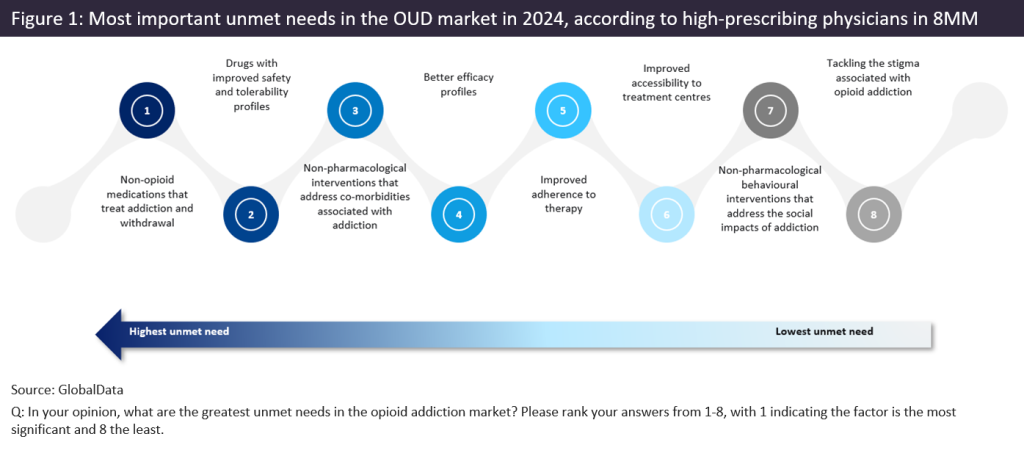
Of the current treatment options for OUD, key opinion leaders (KOLs) interviewed by GlobalData and surveyed prescribing physicians agreed that there is a gap in the market for safer non-opioid medications that treat addiction and withdrawal. KOLs cast doubt over the safety profile of methadone, citing that it prolongs the QT interval (the term “long QT” refers to the heart’s electrical activity as graphed on an ECG [electrocardiogram]), which creates a higher risk for fatal arrhythmias. Furthermore, a common theme raised by KOLs was that drug regulators are concerned about “diversion”—the illicit use of opioid medications. KOLs referenced the fact that methadone is frequently sold on the street by OUD patients participating in methadone treatment programs. Additionally, certain formulations of buprenorphine have naloxone (an opioid antagonist) added to deter illicit use.
According to GlobalData’s Drug Database, six out of the seven agents currently in late-stage development (Phase IIb–III) are non-opioids. This includes a cannabinoid product, as well as a PPARγ agonist, a sodium-dependent dopamine transporter inhibitor, an androgen receptor antagonist, and a pannexin-1 inhibitor. KOLs are sceptical regarding the ability of these therapeutic candidates to replace methadone and buprenorphine as first-line treatments. Jazz Pharmaceutical’s Epidyolex is the only asset with published Phase II outcomes in a study evaluating its efficacy as an adjunct treatment to patients on opioid agonist therapy (NCT04567784). The study was conducted by an academic institution, the Icahn School of Medicine at Mount Sinai, rather than by Jazz as part of a wider commercialisation effort for Epidyolex in OUD. The results suggest that cannabidiol (Epidyolex) is an effective and well-tolerated pharmacologic intervention as an adjunctive treatment to medication-assisted therapy (MAT) to reduce the risk of relapse. Epidyolex and other cannabidiol products may enter the market as adjunctive treatments to the existing first-line therapies, but this would not likely displace methadone or buprenorphine as first-line treatments for OUD. There is a lack of efficacy data at this time for the other pipeline agents mentioned. Therefore, despite the presence of non-opioids in the pipeline, high-efficacy non-opioids remain an exploitable opportunity.
Any novel treatments for OUD will have to demonstrate significantly improved efficacy to displace the gold standards of treatment, methadone and buprenorphine. Both methadone and buprenorphine are widely available in different formulations and are well-recognised by OUD patients. GlobalData expects that any non-opioid products developed for the treatment of OUD would likely see strong uptake and could potentially alter the OUD market landscape.
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Your download email will arrive shortly
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalData
