Want to stay on top of the science and politics driving biotech today? Sign up to get our biotech newsletter in your inbox.
Hiya. Once more, a bill has been introduced in Congress to incentivize small molecular drug development by removing what the industry calls the Inflation Reduction Act’s “pill penalty.” Also, a startup run by Merck alum Roger Perlmutter is raising $350 million.
advertisement
Roger Perlmutter’s Eikon raises $350 million
From STAT’s Matthew Herper: Eikon Therapeutics, the cell-biology-focused startup run by former Merck R&D head Roger Perlmutter, announced this morning that it raised another $350.7 million to fund its drug development efforts. That brings the total amount raised by the company to $1.1 billion.
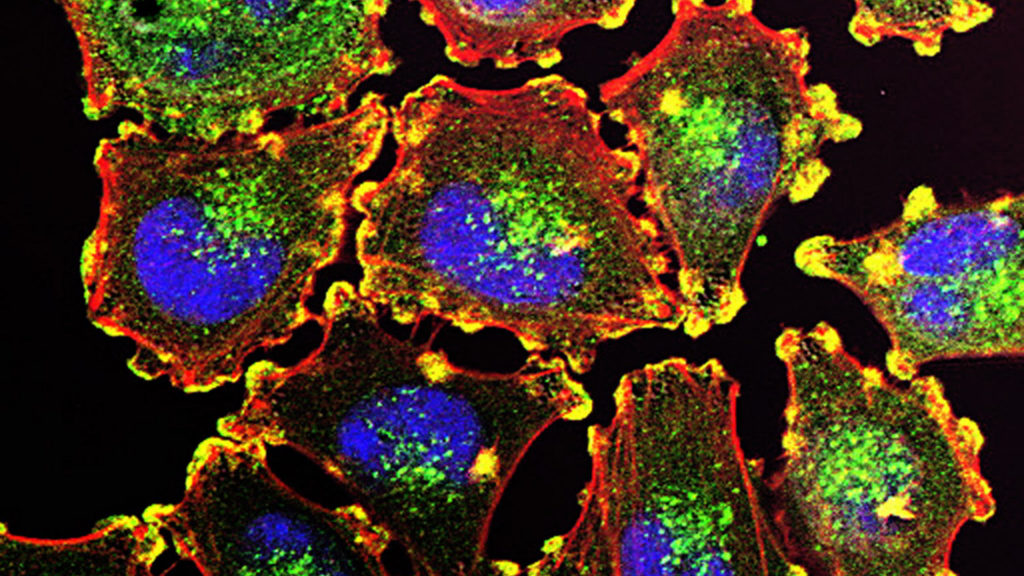
Eikon’s scientific platform uses high-end microscopy to run drug screens that other companies can’t. Much of the new money will go to fund the clinical development of drugs for cancer. Eikon’s lead program leverages the autoimmune expertise Perlmutter and clinical development head Roy Baynes gained building the Keytruda franchise at Merck. It’s an immunotherapy that hits two targets known as toll-like receptors, and is currently being investigated in a Phase 3 study for melanoma. Eikon is also developing two PARP inhibitors, also for various forms of cancer, that were licensed from a Chinese firm. The second of these drugs is able to enter the brain, and so could be useful against brain tumors and brain metastases.
The financing round included both new and existing investors, including Lux Capital, Alexandria Venture Investments, AME Cloud Ventures, and many others.
advertisement
A bill is re-introduced to revise IRA provision
Once more, legislation has been introduced in Congress to address the Inflation Reduction Act’s “pill penalty,” as the industry refers to it, which allows small molecule drugs to be up for Medicare price negotiation four years faster than biologics. The Ensuring Pathways to Innovative Cures, or EPIC Act is meant to ensure continued investment into pill-form medicines.
“As a life science investor, the most glaring error in the law, from our perspective, is a false and arbitrary difference in reward based on the type of drug,” one venture capitalist told STAT’s Ed Silverman.
Not everyone agrees. “I believe the industry still has substantial incentives to develop small molecule drugs,” said Stacie Dusetzina, a professor of health policy and an associate professor of cancer research at Vanderbilt University.
The impact of pulling USAID funding for an HIV trial
A Phase 1 trial in Africa for two experimental HIV vaccines was abruptly halted after USAID froze $45 million in funding, leaving researchers scrambling for alternatives. STAT’s Katherine MacPhail spoke with Glenda Gray, chief scientific officer at the South African Medical Research Council, who leads the BRILLIANT Consortium — the research partnership that led the trial.
“A lot of people who get this funding are academics and NGOs,” she told STAT. “We’re not for-profit, so we don’t have cash flow and an endless supply of money to bridge this gap.”
South Africa has the world’s highest HIV burden and relies heavily on global aid — and the implications of stopping this funding could lead to increased transmission, treatment disruptions, and worsening public health outcomes.
Madrigal drug shows promise in severe MASH patients
From STAT’s Adam Feuerstein: Madrigal Pharmaceuticals said this morning that its MASH drug Rezdiffra showed a potential benefit for patients with a compensated cirrhosis, a severe form of the liver disease with few treatment options outside of a liver transplant.
In an open-label arm of a previously reported Phase 3 study, patients with a diagnosis of compensated cirrhosis due to MASH who were treated with Rezdiffra for two years achieved a statistically significant reduction in liver stiffness, measured by a non-invasive imaging test.
advertisement
Liver stiffness is a surrogate marker for fibrosis, or scarring, of the liver that can only be measured with a biopsy. Madrigal didn’t conduct biopsies on the patients in this study, but will in a separate and ongoing Phase 3 study.
Rezdiffra is currently approved to treat patients with early and moderate MASH. Sales in the fourth quarter, also reported this morning, were $103.3 million.
More reads
-
Emalex dopamine blocker stops Tourette syndrome relapse in phase 3 win, FierceBiotech
-
Candel’s viral therapy shows promising survival in small pancreatic cancer study, Endpoints