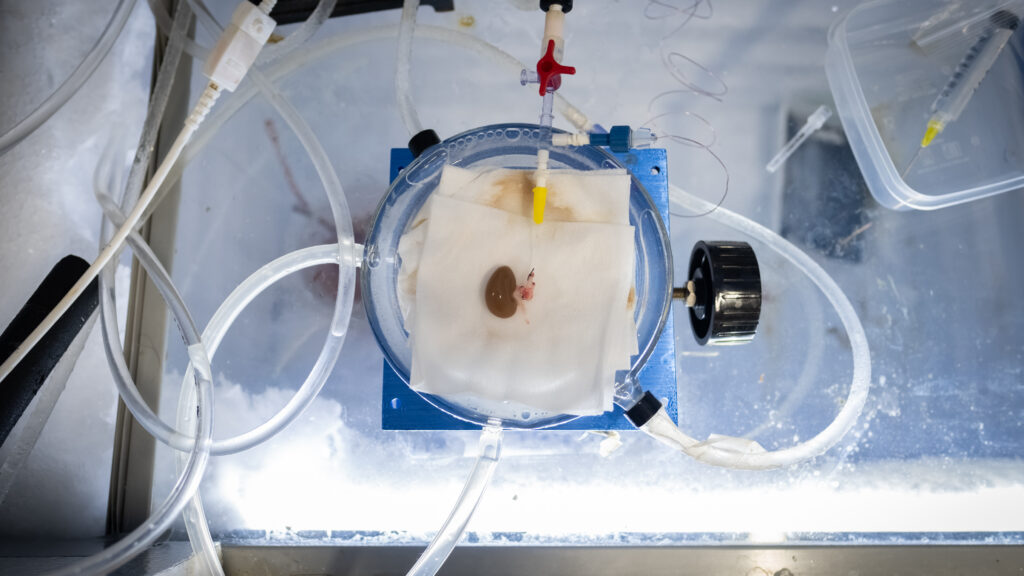
The rat kidney was peculiarly beautiful — an edgeless viscera about the size of a quarter, gemstone-like and gleaming as if encased in pure glass.
It owed its veneer to a frosty, minus 150-degree Celsius plunge into liquid nitrogen, a process known as vitrification, that shocked the kidney into an icy state of suspended animation. Then researchers at the University of Minnesota restarted the kidney’s biological clock, rewarming it before transplanting it back into a live rat — who survived the ordeal.
advertisement
In all, five rats received a vitrified-then-thawed kidney in a study whose results were published this month in Nature Communications. It’s the first time scientists have shown it’s possible to successfully and repeatedly transplant a life-sustaining mammalian organ after it has been rewarmed from this icy metabolic arrest. Outside experts unequivocally called the results a seminal milestone for the field of organ preservation.
“It’s historic,” said Mehmet Toner, a biomedical engineer at Massachusetts General Hospital and a Harvard Medical School professor working in organ cryopreservation. “This is the beginning of a very exciting journey.”
If researchers someday successfully translate those results into humans, experts told STAT, organ cryopreservation and rewarming could revolutionize transplant medicine — and potentially save tens of thousands of lives each year in the U.S. alone.
At any given moment, about 100,000 adults and children in the U.S. await a replacement organ. Last year, 41,225 got them. Each was dictated by time above all else: right now, doctors must sprint to obtain and then transplant organs within the tight, hours-long window they can stay alive outside the human body.
advertisement
“Pretty clearly the impact would be enormous,” said David Klassen, chief medical officer for the United Network for Organ Sharing, a non-profit that contracts with the federal government to oversee the U.S. transplant system. “If you remove time from [the equation], then all of a sudden things change dramatically.”
Though the rewarmed rat kidneys suffered some damage in the vitrification and rewarming process, the University of Minnesota team said that it could begin refining and testing the technique on human organs in as soon as the next year or two; clinical trials, they said, could take another five years or more. But scaling up from rats to humans will take another scientific breakthrough, outside experts cautioned — and even then, it’s not clear how cryopreserved organs will perform compared to their fresh counterparts. Transplant clinical trials, moreover, are known to be uniquely challenging, Toner and other outside experts said. “These are very complicated trials,” he said. “I would use caution jumping from here to frozen human kidneys off the shelf.”
Even if they nail the science, broadly rolling out a nationwide network of cryopreserved organ banks will take time and raise ethical questions about who receives which kind of transplant, said New York University bioethicist Art Caplan. “The creation of this pipeline will reawaken some questions about what is the role of commerce in our organ transplant system.”
Suspended animation has long been the stuff of fantasy — a necessity for interstellar journeys, a magical curse, a time-bending gift. It has appeared across myths like Sleeping Beauty, in tales by Edgar Allen Poe, and in many on-screen stories including Avatar, Austin Powers, and the Twilight Zone.
“When I was a Ph.D. [student], I looked at this and I thought, ‘I’m not sure we’re going to get there,’” said John Bischof, a mechanical engineer at the University of Minnesota’s Organ and Tissue Preservation Center and co-author of the Nature paper. The recent study renders that science fiction-esque dream more possible. “There’s hope we can actually do this,” he said. “We can stop biological time.”

In nature, a special cadre of creatures can survive subzero temperatures for weeks or longer. Wood frogs freeze and thaw with their surroundings. Arctic ground squirrels regularly let their body fluids supercool to minus-3 degrees Celsius. In 2021, rotifers took it to the extreme: the microscopic lifeform successfully reproduced after spending 24,000 years locked in Siberian permafrost.
Scientists have attempted to recreate this cold-hardiness since at least the 1930s when a Swiss priest experimented with vitrifying moss and chick embryo hearts.
But it’s one thing to vitrify an organ. It’s another to get it pumping again.
For decades bringing organs back from a deep freeze without injury and with full function has remained a frustrating and unbudging problem for the field. “There have been a lot of false starts,” Bischof said. But the University of Minnesota team has begun to succeed where so many others have foundered.
It’s already vitrified and revived human, mouse, and pig pancreas islet cells, as well as rat hearts and livers. In one remarkable, yet humble, 2020 experiment, two (out of 223) vitrified and rewarmed zebrafish embryos lived on to respawn. “So far we’ve been able to stay on the successful side of failure,” said Erik Finger, a co-author of the new study and transplant surgeon at the University of Minnesota’s medical school.
When vitrifying, scientists first infuse the organ or tissue with magnetic nanoparticles and safeguarding chemicals called cryoprotective agents that serve as a kind of antifreeze. Afterward, they cool it quickly — 24 degrees Celsius per minute — to bypass the formation of cell-shredding ice crystals and directly enter a glass-like state.
Then comes the real challenge. “You need to come back from that,” Bischof said. And when you do, “you have to outrun the ice.”
Bischof and his colleagues have spent years developing technology that can rewarm vitrified materials fast enough to avoid ice-crystal formation in the physical transition back from glass. This rewarming, critically, also must be uniform, to avoid an organ cracking and splitting from its outside surfaces being too different a temperature from its core — like an ice cube in a glass of room-temperature water.
Their solution is a technique called nanowarming, which utilizes a radio-frequency copper coil to create a magnetic field that excites iron nanoparticles throughout the organ all at once, similar to a microwave oven, but more uniform.
During routine human kidney transplants, Finger pays close attention to visual cues of success — a transplanted kidney that quickly “pinks up” and is firm and smooth-colored, not soft and blotchy. “Pretty surprisingly,” he said, “that’s what we saw in rats.”
That’s not to say the nanowarmed kidneys performed exactly like any other. “They worked — but they didn’t work perfectly,” Finger said. The experimental kidneys produced urine within 45 minutes of transplantation, compared to a few minutes for their fresh counterparts. And for the first days after surgery, they were slower to clear out creatinine, a chemical waste product that kidneys remove from the body. Though “by three weeks, they look like normal kidneys,” Finger said.
Gregory Fahy, president of the Society for Cryobiology and executive director and chief scientific officer for 21st Century Medicine, a California-based cryobiological research company, said that initial dysfunction and the three-week recovery time concerned him. “The biggest issue is that the kidneys were, in fact, badly damaged,” said Fahy, who was not directly involved in the research. “The function of those kidneys was cut in about half.”
He and other outside experts pointed out that the rat subjects were relatively young — between four and eight months old — which may not be representative of the health of actual human kidney donors (who are often recently deceased) or recipients (who have chronic and irreversible renal disease). “These were kidneys in the peak of life, in perfect health — and they barely made it,” Fahy said. “I think if they’d been any more damaged than they were they wouldn’t have made it.”
On the other hand, he said, the degree to which the kidneys did heal and recover was “remarkable and encouraging.” In the paper, the researchers also noted that because they ended the study 30 days post-transplant, they weren’t able to assess longer-term survival.
The University of Minnesota researchers said they plan to spend the next six months attempting to scale their cryopreservation method up to pig organs — a size change, kidney-wise, from a large grape (in rats) to about a pear (in pigs). As they go, they will continue to study whether rewarmed animal organs recover their original physiological, chemical, and electrical properties.
Down the line, if all goes well, the future might hold living banks where organs, skin, nerves, blood vessels, cartilage and stem cells are preserved in liquid nitrogen for years until they’re matched with the right patients.
But that vision still remains many years away. Toner, at Mass General, said scaling up from a rat to a larger animal presents a major hurdle. “There’s not just the size and shape but the biological side as well,” he said, like differences between each animal’s blood composition and immune system. “If animal models were perfect, we would’ve cured cancer.”
If the Minnesota team overcomes this challenge, they will then have to design a complex human clinical trial for the Food and Drug Administration — which might consider the technology both a drug and device. “It’s a major effort,” said Paolo Fontes, who spent 30 years in transplant surgery and medicine at the University of Pittsburgh and is now a chief medical scientific officer for Eikonoklastes, a biopharmaceutical company. “To run clinical trials in transplantations is so hard,” he said.
For example, it could be difficult to source the kidneys for human trials of vitrification, given how precious and under-supplied they already are for people awaiting regular transplants. “Why would you freeze it when you could have used it fresh?” Toner said. And if they use kidneys deemed unusable for traditional transplantation, it could discourage patients from enrolling or skew the final results. “It might destroy the whole trial,” Fontes said.
Ultimately, the technology’s success will depend on more than making it through clinical trials. “It’s not necessarily enough to say the organ will support life — it’s whether the doctor will be willing to put it in his or her patient,” Fahy said. “There are many unanswered questions for how to scale this up.”


Transplant medicine has come a long way since the first kidney was successfully transplanted in 1954. “It’s not magic anymore,” Toner said. “It’s a supply chain management issue.” And a time management issue.
Stored on ice — transplant medicine’s current preservation standard — hearts and lungs can last six or eight hours max before lack of blood and oxygen sets in. That timeframe goes up to 12 hours for livers, 18 hours for pancreases, and 36 hours for kidneys.
This race against the clock means organs are often hustled by helicopter or airplane — a risky strategy in more ways than one. For starters, commercial travel delays lead to lost and spoiled organs. And ultra-urgent aeromedical transport poses a danger to clinicians: by one estimate, the risk of fatality while traveling on an organ procurement flight was 1,000 times higher than on a scheduled commercial flight.
“It’s all an emergency,” said Julie Kemink, a former surgical nurse and chief operating officer of LifeSource, an organ procurement organization that coordinates transplants for Minnesota, North Dakota, South Dakota and parts of Wisconsin. “We feel the time crunch. It’s like planning a wedding in 48 hours.”
When each individual transplant has to be treated as a crisis, experts say, recipients don’t always get adequate time to prepare, medically or emotionally. Their surgical teams, too, have to scramble into action. It also means the organ matching process prioritizes people living closer to donors — just one of many inequities baked into the country’s current transplant system.
People of color, individuals of lower socioeconomic status, residents of rural areas, undocumented immigrants, and individuals with intellectual disabilities all struggle to make it onto organ transplant waitlists, according to a 2022 report by the National Academies of Sciences, Engineering, and Medicine. The report also notes that Black candidates, in particular, are more likely to develop kidney failure than white patients but wait twice as long and are ultimately much less likely to receive a transplant. Mental illness or substance use can be held against people waiting for transplants, said Caplan, the NYU bioethicist, as can a doctor’s subjective perception of a patient’s likelihood to follow directions. “People tend to look for reasons to exclude when they know the list is long,” he said.
The current system also fails to make the most of potential donations. Only 0.3% of people who die in the United States become organ donors, and many organs that are procured from donors never make it into a recipient. Overall, about 17 people die each day waiting for a transplant. “Right now, without question, the supply of organs does not meet demand,” Klassen said.
The recent advances in cryopreservation also come at a time when the national organ transplant system is under intense scrutiny. Last August, a 2½-year U.S. Senate investigation revealed transportation issues, out-of-date coordination technology, minimal oversight, and numerous testing and logistical errors that have resulted in transplant recipients dying or being harmed. In March, the Biden administration announced it would revamp the country’s transplant system.
Transforming transplantation from an emergency to a semi-elective procedure would offer advantages from procurement to recipient recovery, said William Chapman, a transplant surgeon at Washington University in St. Louis who is not involved with any cryopreservation research. “It’s still early on, but I think it has potential,” he said. “It would totally change the landscape.”
With more time, recipients could receive pre-treatments to improve their immune system’s tolerance of a donor organ. They could also be more carefully matched to reduce the risk of rejection, said Susan Wolf, a bioethicist at the University of Minnesota who advises its cryopreservation research center. People living in remote or rural areas, as well as patients with limited mobility, would be able to plan ahead for traveling to scheduled appointments, she added.
Clinicians could more thoroughly screen organs for malignancies and transmissible diseases such as rabies and HIV (disease transmission rates for organ transplantation are less than 1%, but still about 10,000 times higher than in blood transfusion, where a weeks-long shelf life allows for in-depth testing). They would have more time to book operating rooms, assemble staff, and ensure they have enough blood supply. “Safety would improve,” Chapman said. And as for the vexing issue of unused donor organs, “You wouldn’t have to see them thrown away,” Caplan said. “You could build up a reservoir, a supply.”
But the field would have to confront new issues, too — whether doctors will be able to accept any difference in quality between fresh and vitrified-then-thawed organs. “It’s unlikely it’s going to be better than fresh,” Finger said. “How do you decide how good it has to be?” Even with regulatory approval, he said, “clinicians are still going to be a little wary.”
Some might want to hold vitrified organs to the same quality standard as fresh ones, Chapman said, while other surgeons might make the comparison, instead, to the health and mortality risk of waiting for a transplant indefinitely. “Even though the organ may not function as well or last as long, would that serve a need that currently isn’t being met? Maybe,” Chapman said. “It’s a tough judgment call.” Scientists will also have to determine how long a vitrified organ can be stored. “How far past the expiration date are you willing to go?” Caplan said.
And as the infrastructure for organ banks develops, new inequities will face would-be recipients. For one, if organ banking becomes a for-profit commercial industry, then it is likely that wealthier and well-insured patients will be the first to benefit, Caplan said. Even if they function as nonprofits, patients geographically closer to them will be advantaged. “Some areas are going to be serviced by a bank before others,” he said. “They’re not going to be available tomorrow morning, everywhere, equally.”