Dive Brief:
- Synchron, a startup making a brain implant rivaling Elon Musk’s Neuralink, is launching a patient registry.
- The New York-based company is developing technology that would allow people to use computers and other devices with hands-free point-and-click. The idea has garnered backing from Bill Gates’ and Jeff Bezos’ investment firms.
- Synchron said last year that six U.S. patients were implanted with its Stentrode device in a feasibility study. The company declined to comment on the timing of the results. CEO Thomas Oxley told Reuters on Monday that the company is preparing to recruit patients for a large-scale clinical trial that would be required to seek device approval from the Food and Drug Administration.
Dive Insight:
Brain-computer interface (BCI) technologies, such as Synchron’s implant, translate brain signals to allow users to operate digital devices. The technology is still largely considered experimental, and developing it can be challenging because each person generates unique brain signals, the U.S. Government Accountability Office said in a 2022 report.
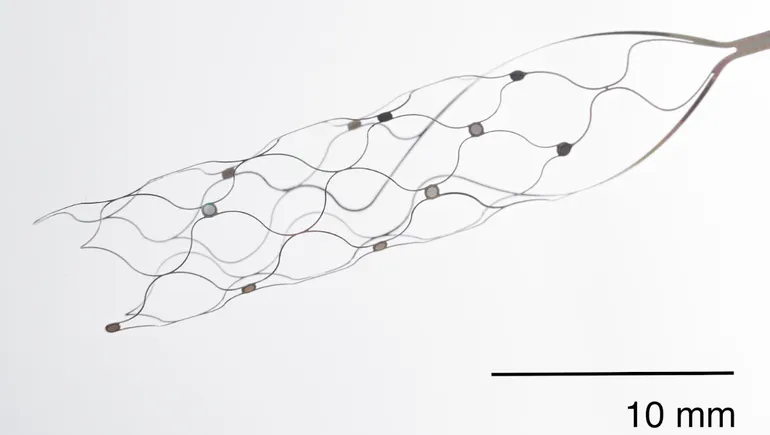
Synchron’s Stentrode device is intended to help people with limited mobility operate technology using only their thoughts. It stands apart from competitors because the procedure to place the device doesn’t require open brain surgery. Placement is done through a minimally invasive procedure where the device is implanted through the jugular vein and sits atop the blood vessel on the surface of the motor cortex, the part of the brain responsible for movement.
Once in place, Stentrode expands to press electrodes against the vessel wall close to the brain, where it can record neural information and deliver currents to targeted areas, a Synchron spokesperson wrote in an email. The signals are captured and sent to a wireless antenna unit implanted in the chest, which sends them to an external receiver.
A patient using the technology would also need to learn how to control a computer operating system that interacts with assistive technologies.
Synchron will analyze the preliminary data from the feasibility study while waiting for the FDA’s blessing to proceed with a larger study, CEO Oxley told Reuters. The company plans to include “dozens of participants” in the trial and has received interest from about 120 clinical trial centers, he said in the report.
Neuralink, which is also working on brain chips, said last year that it had received FDA approval to begin its first-in-human study. However, FDA inspectors found problems with record keeping and quality control for animal experiments less than a month later, Reuters reported. Musk tweeted in January that the first patient received an implant and posted a video last month of the person playing chess on a computer.
Other BCI companies include Blackrock Neurotech, which received a breakthrough device designation for its system, and Braingate, which is running a feasibility study.
Mass General Brigham launched an industry group last month focused on BCI technology. The group, which will collaborate with the FDA’s Center for Devices and Radiological Health, includes Synchron, Neuralink and Blackrock Neurotech.