During my 25 years working in clinical trial operations, I’ve seen the biopharmaceutical world talk a big game about making the process easier for the sites running the trials and the patients participating in them. Everyone from trial sponsors to the Food and Drug Administration has been quick to promise simpler processes, less hassle, and better experiences for everyone involved.
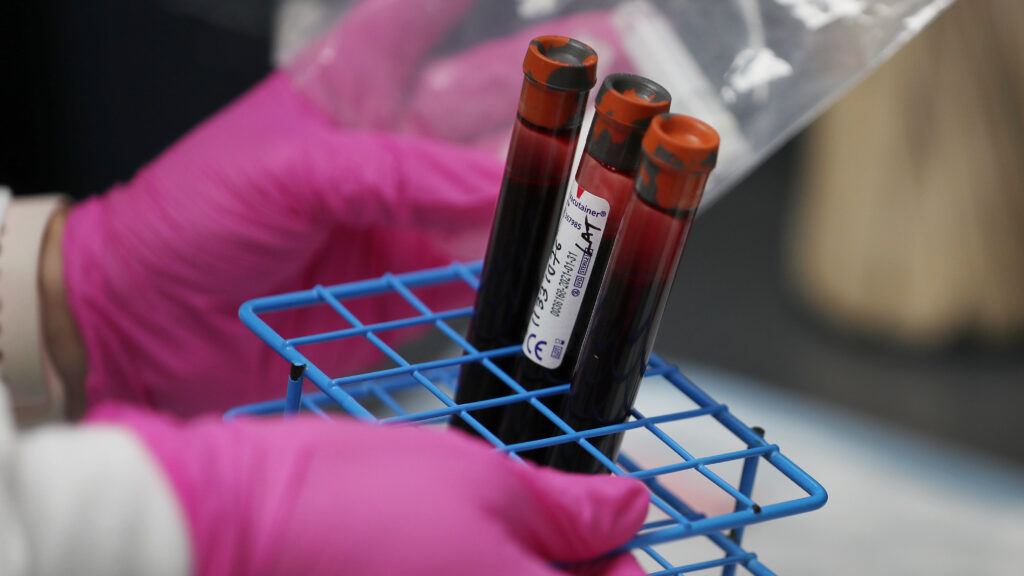
But look closer, and you’ll see that these grand promises often fall apart when sponsors implement complicated plans for collecting blood, tissue and other biospecimens and impossible processes for managing those samples. Behind all the talk and regulatory guidance, there’s a reluctance to change how these essential elements of clinical trial execution are handled, putting the very ideals that sponsors are trying to uphold at risk.
advertisement
The concept of site and patient centricity frequently gets reduced to addressing only superficial gestures, such as committing to promptly paying sites for their work or using tablets for conducting patient surveys. But the essence of centricity extends far beyond these surface-level measures. It lies in addressing the unseen and unmentioned complexities of clinical trials and providing real solutions that focus on truly prioritizing the needs of trial sites and participants.
STAT+ Exclusive Story
Already have an account? Log in

Get unlimited access to award-winning journalism and exclusive events.