Want to stay on top of the science and politics driving biotech today? Sign up to get our biotech newsletter in your inbox.
Good morning. Let’s get straight into another busy news day.
advertisement
The need-to-know this morning
- Novartis said it will acquire Anthos Therapeutics, the developer of a new type of blood-thinning medicine, for $925 million, or as much as $3.1 billion if certain future conditions are met. Novartis and Blackstone Life Sciences co-founded Anthos in 2019 to develop abelacimab as a potential treatment to prevent strokes and blood clots in patients with certain heart conditions.
Judge halts cuts to NIH funding
Here’s a recap of the fast-moving developments with NIH funding:
Last Friday, the NIH announced that it would immediately cut funding for indirect costs paid to research institutions, capping the rate of support to 15% of grants. The move sent shockwaves through the science world, with some universities and medical each standing to lose $100 million a year or more.
Yesterday, attorneys general (all Democrats) representing 22 states sued the Trump administration over the move. Within hours, a federal judge in Boston ordered a nationwide temporary pause on plans by the National Institutes of Health to substantially slash research overhead payments to universities, medical centers, and other grant recipients.
advertisement
Two more lawsuits were filed later on Monday, on behalf of private universities and hospitals, seeking to block implementation of the policy nationwide. Some key Republican senators also began pushing back on the NIH cuts.
Indirect costs are administrative, facility, and other expenses not directly linked to the goals of a scientific project. They include costs to keep the lights on in labs, to pay for water and heating, and to keep equipment in working order. For an explainer on indirect cuts and the Trump policy, go here.
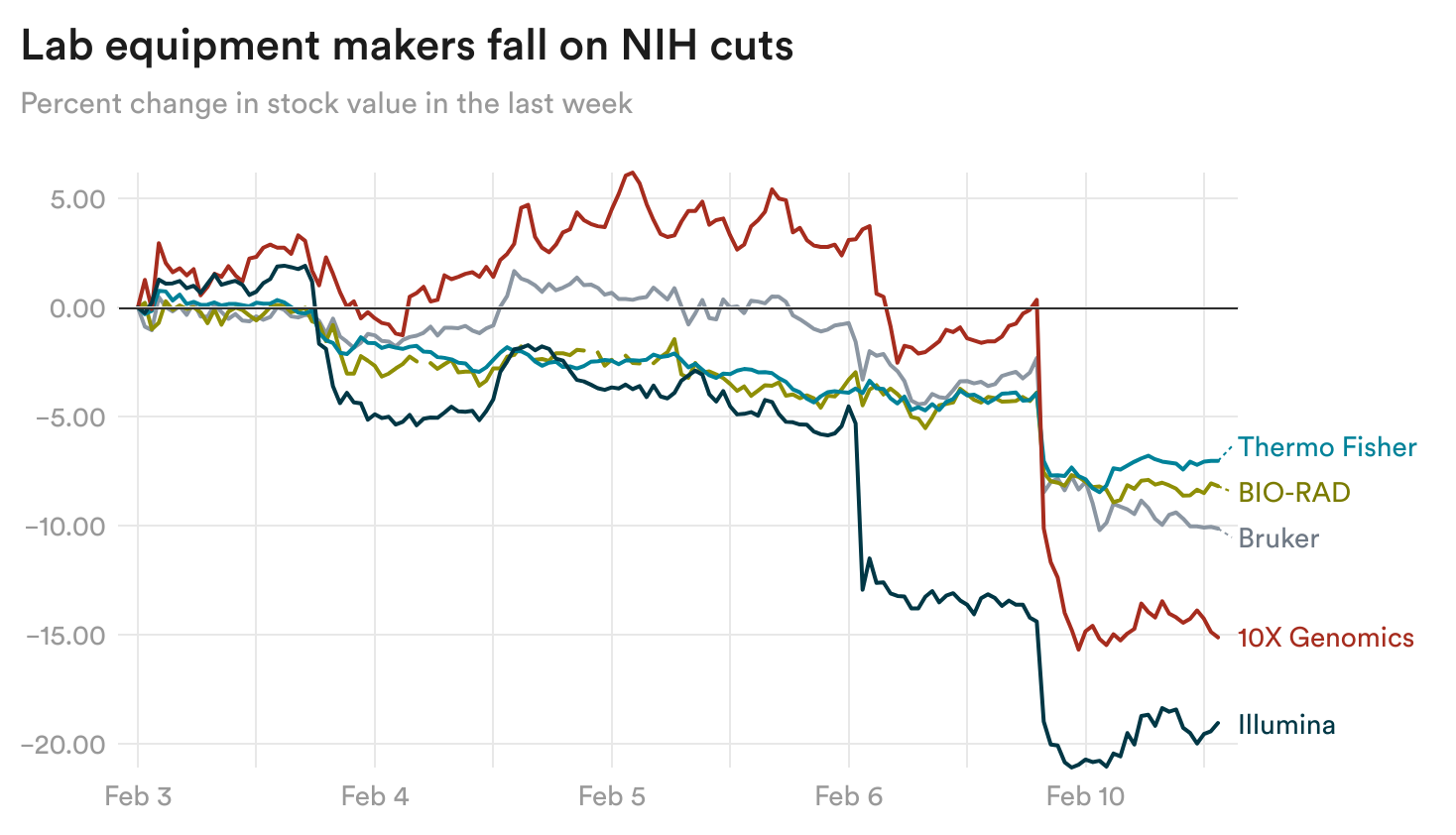
Shares of companies that make equipment and tools used in labs, including Illumina and 10x Genomics, sank yesterday amid concerns about the NIH cuts.
RFK Jr. could revive a defunct vaccine task force
Robert F. Kennedy Jr., who is set to be confirmed to lead HHS in a matter of days, may revive a dormant vaccine task force as a way to scrutinize vaccinations, my colleagues Sarah Owermohle and Rachel Cohrs Zhang report.
Congress created this task force almost 40 years ago to recommend ways to make childhood vaccines safer, but it’s been defunct since 1998. For several years now, RFK’s allies have zeroed in on the panel and made misleading statements about its work to build a narrative about the government’s inaction on the safety of childhood vaccines.
For example, Del Bigtree, who has advised RFK Jr., claimed that the task force had never issued reports required by Congress about how to make vaccines safer. But the task force did in fact produce a comprehensive report that is public — and which STAT found via a Google search.
Vertex pain pill dominates earnings call discussion
From my colleague Adam Feuerstein: If you’re curious (and I was) Vertex Pharmaceuticals’ newly approved acute pain drug, Journavx, is pronounced “JUR-nav-ex.” Plans for the pill’s commercial launch, which starts in March, dominated the discussion during Vertex’s earnings call last night. Executives expressed confidence that Journavx’s non-addictive properties — and the need to further reduce the use of opioids — will convince hospitals, surgeons, and insurers to prescribe the new pain treatment, despite its relatively high cost. Analysts on the call had many, many questions.
advertisement
Vertex didn’t offer any sales projections for Journavx this year, but said the pill factored into its overall forecast for revenue in the range of $11.75 to $12 billion — guidance that’s in line with Wall Street’s consensus, which includes $86 million in Journaux sales, according to Visible Alpha.
Vertex also announced Chief Operating Officer Stuart Arbuckle would retire in July after 12 years with the company. Charlie Wagner, CFO, will assume the additional role of COO, and Duncan McKechnie, currently head of commercial operations in North America, will be promoted to chief commercial officer.
Novo counters Hims’ ad with its own ad
If you watched the Super Bowl, you may have caught a commercial from telehealth company Hims that promotes its compounded GLP-1 drugs. We’ve previously reported how experts were concerned that the ad — which cast pharma companies as being part of an industry that puts profits over patients — failed to disclose the drugs’ possible side effects. Some lawmakers even called on the FDA to probe the commercial.
Novo Nordisk, maker of branded GLP-1 treatments, has now countered with an ad of its own.
The ad, placed in major newspapers, urges people to “check before you inject,” warning them that compounded GLP-1 drugs are not FDA-approved and not proven safe or effective. In a statement, Novo said, “the recent explosion in irresponsible and misleading advertising about compounded GLP-1 drugs, like the Hims & Hers Super Bowl commercial, puts patients’ safety at risk and we cannot stay silent.”
Pharma companies have been in a long-standing battle against compounders and the telehealth companies they work with. Eli Lilly is currently part of a lawsuit filed by a compounding trade group that concerns whether compounders can continue to make copies of Lilly’s GLP-1 drugs.
More reads
- HIV infections could jump over 6 times if US support is dropped and not replaced, UNAIDS chief says, Associated Press
- Mass General Brigham announces largest layoff in its history amid ongoing restructuring, The Boston Globe

